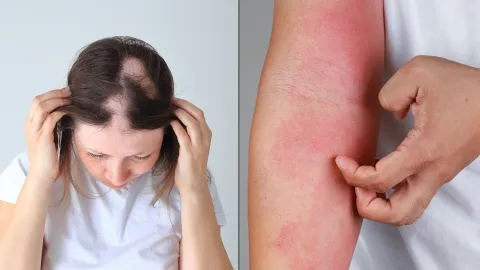
This study is testing whether a medicine called abrocitinib can help people 12 years and older with Down syndrome treat alopecia areata (hairloss) and symptoms of atopic dermatitis (eczema).
Who can participate in this study?
- Individuals with Down syndrome at least 12 years old
- Have a diagnosis of alopecia areata, which affects at least 25% of the scalp AND/OR a diagnosis of atopic dermatitis, which affects at least 7% of the body
Who cannot participate in this study?
- Individuals without Down syndrome
- Individuals younger than 12 years old
- Have a history of tuberculosis, hepatitis or HIV
- Have a diagnosis of other inflammatory skin condition (e.g., psoriasis, seborrheic dermatitis, lupus)
- Are currently receiving another treatment for alopecia areata or atopic dermatitis
Contact research team for additional eligibility requirements.
Where does this study take place?
New York, NY
What activities are involved in this study?
- Blood tests
- Pregnancy test (if it applies)
- Skin checks
- Physical exams (checking weight, temperature, blood pressure)
- Surveys
- Photos
- Tape strip tests (only for people with eczema)
- Optional skin biopsies
What is the expected time commitment of this study?
Participation in this research study is expected to last approximately 64 weeks.
What languages does this study support?
English
Is compensation provided?
Yes
If interested in participating in the Skin and Scalp Diseases in Individuals with Down Syndrome Clinical Trial or for more information, please contact Giselle Singer at (212) 241-3288 or giselle.singer@mssm.edu.